'Diamond Planets' Crappy For Life (As We Know It)
There are few celestial objects that inspire such vivid imaginings than carbon-rich planets. Carbon, of course, is the key ingredient in diamonds. So if you follow the logic, carbon-rich alien worlds may evolve into exotic planets with spires of diamonds jutting out of the surface, or layers of diamond-rich gravel, glistening in the starlight.
However, theoretical models of these carbon-rich worlds conclude that ‘diamond planets’ probably aren’t life’s best friend.
In research presented at the American Astronomical Society Division of Planetary Sciences meeting in Denver, Colo., on Oct. 7, Torrence Johnson of NASA’s Jet Propulsion Laboratory in Pasadena, Calif., highlighted the challenges life (as we know it) would face in carbon-rich planetary systems.
“The building blocks that went into making our oceans are the icy asteroids and comets,” he said. “If we keep track of these building blocks, we find that planets around carbon-rich stars come up dry.”
The problem, according to Johnson and his colleagues, is that the extra carbon found in carbon-rich proto-planetary systems would lock onto oxygen, preventing the oxygen from chemically bonding with hydrogen. Of course, the key substance for life on Earth is water, a.k.a. H2O — two hydrogen atoms combined with one oxygen atom. It seems that, in this scenario, life would be snuffed-out before it could even begin.
“It’s ironic that if carbon, the main element of life, becomes too abundant, it will steal away the oxygen that would have made water, the solvent essential to life as we know it,” said research collaborator Jonathan Lunine of Cornell University, Ithaca, New York.
We take water for granted on our planet and throughout the solar system, but “all rocky planets aren’t created equal,” said Lunine.
Our sun was formed by the gravitational collapse of a rich cloud of gas and dust that originated from older generations of stars that exploded as supernova or fizzled out of existence billions of years ago. It just so happens that the carbon-to-oxygen ratio from this stellar gas soup was just right for our sun to form silicate-rich rocky worlds like Earth (as opposed to carbon-rich worlds) with the perfect amount of water ice accumulating beyond the “snow line” of the solar system. The snow line is the distance at which a given substance will freeze, coalesce and accumulate. Asteroids and comets collected their abundance of water in this region — thought to be the source of the liquid water oceans on Earth.
However, in models where the carbon-to-oxygen ratio was varied to favor the formation of carbon-rich worlds, no water formed. In this case, “there’s no snow beyond the snow line,” said Johnson.
This creates an interesting complexity to the search for “habitable” exoplanets. Although a candidate “Earth 2.0″ may be the same size as our planet and orbit within its star’s “habitable zone” — the region surrounding a star where it’s not too hot and not too cold for liquid water to exist on a planetary surface — if it is carbon-rich, it could be the antithesis of “habitable.”
“So-called diamond planets the size of Earth, if they exist, will look totally alien to us: lifeless, ocean-less desert worlds,” Lunine concluded.
Publication: “Planetesimal Compositions in Exoplanet Systems,” Torrence V. Johnson, Olivier Mousis, Jonathan I. Lunine, Nikku Madhusudhan, 2013.
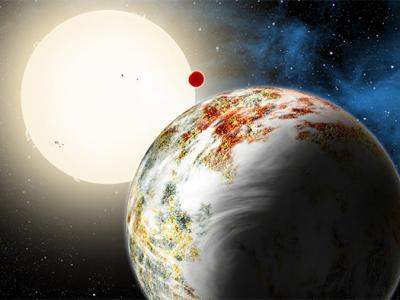
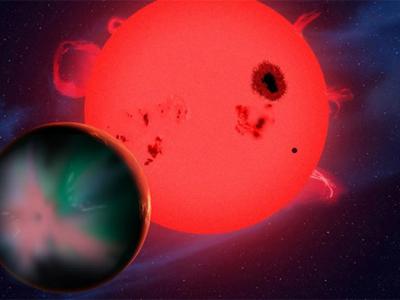
Image: This artist’s concept illustrates the fate of two different planets: the one on the left is similar to Earth, made up largely of silicate-based rocks with oceans coating its surface. The one on the right is rich in carbon — and dry. Credit: NASA/JPL-Caltech(Oct 25, 2013 03:36 PM ET // by Ian O'Neill)